Imaging Syto 60 Stained Nucleic Acid Gels on Odyssey Imagers
Introduction
Syto dyes are cell-permeant cyanine dyes that bind to nucleic acids. Several Syto dyes are available with varying cell permeability, fluorescence enhancement upon binding to nucleic acids, excitation and emission spectra, and nucleic acid selectivity and binding affinity. Syto 60 stain has absorption and fluorescence emission maxima of 652/678 nm.
Nucleic acids stained with Syto 60 stain can be detected and quantified on the Odyssey family of imagers using the 700 nm channel.
In this application guide, three methods are presented for staining serial dilutions of a 1 kb DNA ladder and a 50 bp DNA ladder (New England Biolabs, P/N N3232 and N3236) using Syto 60 stain. This stain can be included in the DNA sample for detection using an Odyssey Imager; it can also be combined with Ethidium Bromide (EtBr) and included in the DNA sample for visualization on an Odyssey Imager and UV transilluminator. Alternatively, it can be diluted and used alone as a post-electrophoresis gel stain.
Methods
The following sections describe three methods for staining DNA with Syto 60 stain.
Method I: Electrophoretic Staining
Purpose: To obtain an archivable, digital image of a DNA agarose gel using an Odyssey Imager.
This method may not be optimal for visualizing bands smaller than 100 bp. See Method III: Post-Electrophoretic Staining for visualizing bands <100 bp.
Method
-
Dilute the Syto 60 stain in TE buffer (1:1,000 dilution). Mix well.
-
Prepare DNA samples in loading dye and reserve an additional 1 µL in the final volume to accommodate the 1 µL of Syto 60 stain for loading.
-
To each sample, add 1 µL of the diluted Syto 60 stain and mix well with a pipettor.
-
Incubate at room temperature (RT) for 5 minutes.
-
Load the samples on the gel.
-
Run the gel at ~5-10 V/cm for approximately 1 hour or less.
-
Use an Odyssey Imager to obtain a digital image of the Syto 60-stained DNA.
Imager Settings
| Imager | Imaging Software | Sample Orientation | Acquisition Time |
| Odyssey M Imager | LI‑COR® Acquisition Software | Sample side up on the Scan Surface | Variable |
| Odyssey DLx Imager | LI‑COR Acquisition Software | Sample side down on scan bed | Variable |
| Odyssey CLx Imager | LI‑COR Acquisition Software or Image Studio™ Software | Sample side down on scan bed | Variable |
| Odyssey XF Imager | LI‑COR Acquisition Software | Sample side up on imaging tray | 2 minutes |
| Odyssey Fc Imager | LI‑COR Acquisition Software or Image Studio Software | Sample side up on imaging tray | 2 minutes |
| Odyssey Sa Imager | Image Studio Software | Sample side down on the Membrane Carrier | Variable |
|
Odyssey Classic Imager |
Image Studio Software | Sample side down on scan bed | Variable |
Imaging Software Settings
LI-COR Acquisition Software:
- Focus offset: 0.5 mm (default)
- 700 nm intensity settings: N/A
Image Studio Software:
- Focus offset:
- Odyssey Sa Imager: 1.7 - 2.0 mm
- Odyssey CLx Imager and Odyssey Classic Imager: 0.5 mm (recommended)
- 700 nm intensity settings: 5-8
Example Data

Method II: Dual Electrophoretic Staining
Purpose: To obtain a digital image using an Odyssey Imager then visualize DNA bands on a UV transilluminator for excision.
This method may not be optimal for visualizing bands smaller than 100 bp. See Method III: Post-Electrophoretic Staining for visualizing bands <100 bp.
Method
-
Dilute Syto 60 stain in TE buffer (1:1,000 dilution). Mix well.
-
Dilute EtBr (10 mg/mL solution) in TE buffer (1:500 dilution). Mix well (made fresh, see Methods I and II: Hints and Tips).
-
Prepare DNA samples in loading dye and reserve an additional volume of 2 µL to accommodate the volume of Syto 60 stain and EtBr for loading.
-
To each sample, add 1 µL of the diluted EtBr and mix with a pipettor.
-
To each sample, add 1 µL of the diluted Syto 60 stain and mix with pipettor.
-
Incubate at RT for 5 minutes.
-
Load the samples.
-
Run the gel at ~5-10 V/cm for approximately 1 hour or less.
Longer run times result in fading of the Syto 60 intensity.
-
Use an Odyssey Imager in the 700 nm channel to obtain a digital image of the Syto 60-stained DNA.
Imager Settings
If using a UV transilluminator, place gel on UV transilluminator to identify bands for excision. If the band(s) to be excised are not bright enough, the gel can be soaked for a short time in a 2 mg/mL solution of EtBr in TAE or TBE buffer after imaging on an Odyssey Imager.
For Odyssey Imager settings, see Imager Settings.
Imaging Software Settings
For imager software settings, see see "Imaging Software Settings ".
Example Data

Methods I and II: Hints and Tips
-
The range of dilution for Syto 60 stain is 1:500 to 1:20,000. The dilution to use is dependent on the DNA size concentration, and whether Syto 60 stain will be used in combination with EtBr.
This method may not be optimal for visualizing bands smaller than 100 bp.
-
Syto 60 stain, diluted within the recommended range in TE buffer, is stable for 1 week at 4 °C.
-
EtBr is not stable in TE and should be diluted fresh each time.
-
The grade of agarose is important. High grade or Molecular Biology grade agarose is less likely to cause “speckling” on Odyssey images.
-
When using an Odyssey DLx Imager, Odyssey DLx Imager, Odyssey XF Imager, Odyssey Sa Imager, or Odyssey Classic Imager to image DNA gels stained with Syto 60 stain, it may be necessary to scan the gel sample side down on the scan bed and/or adjust the focus offset, depending on the gel thickness. A 5-7 mm thick gel is optimum.
-
Addition of EtBr to the gel and running buffer with Syto 60 stain added in the sample is not recommended.
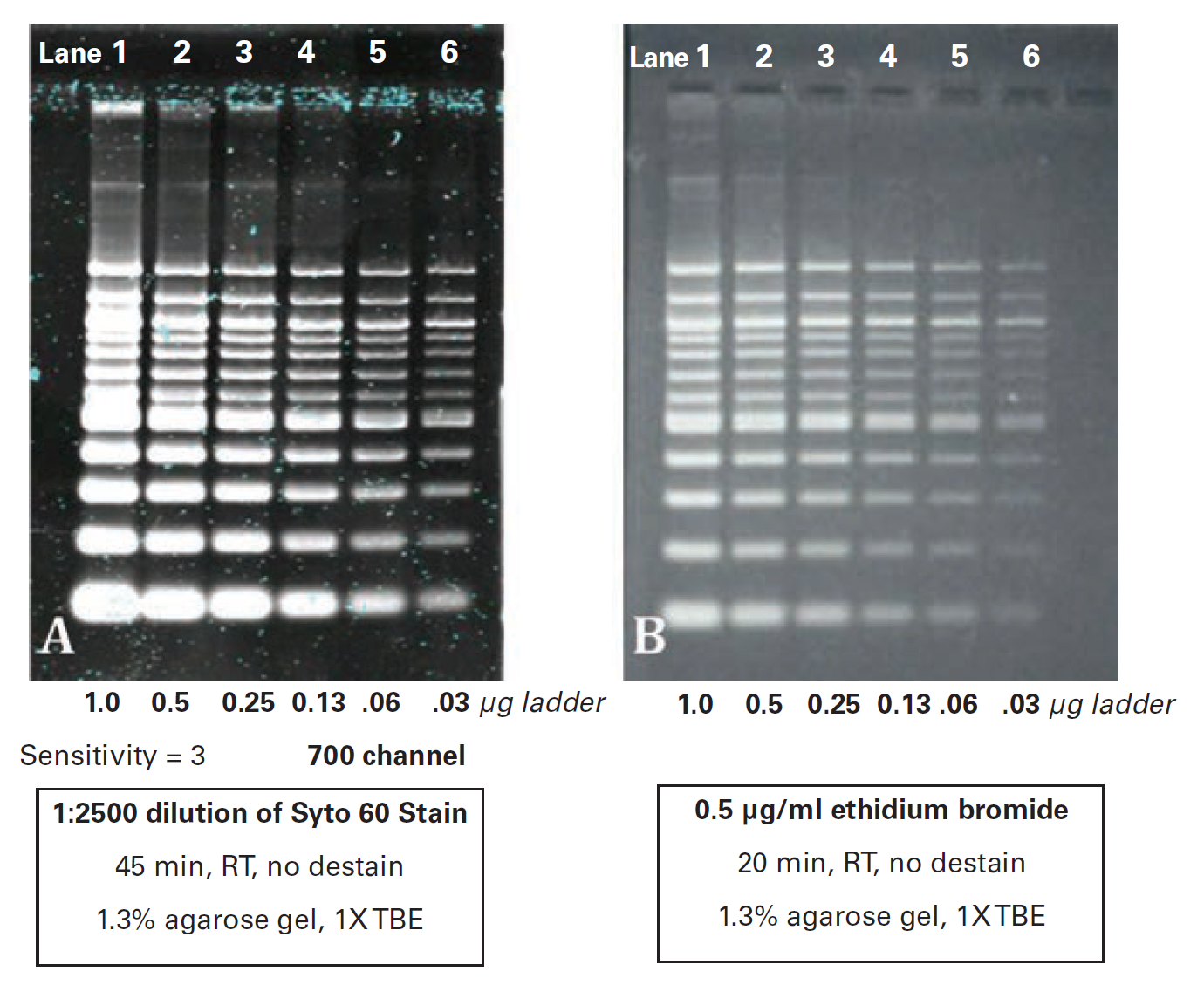
Method III: Post-Electrophoretic Staining
Purpose: To obtain an archivable, digital image of a DNA agarose gel using an Odyssey Imager.
This method is recommended for visualizing bands <100 bp.
Method
- Load two parallel 1.3% agarose/TBE gels with serial two-fold dilutions of 100 bp DNA ladder (New England Biolabs) from 1 µg to 0.3 µg per lane.
- Electrophorese the gels in 1X TBE running buffer at approximately 5 V/cm.
- Stain one gel with Syto 60 dye diluted 1:2500 in water for 45 minutes at RT. Rinse briefly with double distilled water then image in the 700 nm channel using an Odyssey Imager. Use the instrument settings provided in Imager Settings and Imager Settings.
-
Stain the second gel in 0.5 µg/mL EtBr for 20 minutes at RT. Rinse briefly in water then image using a UV transilluminator and a standard CCD camera.
The Odyssey XF Imager and Odyssey XF Imager with 600 nm channel capabilities can also be used to image Ethidium Bromide gels. See the Imaging Nucleic Acid Gels on the Odyssey Fc Imager application note for additional information.
Example Data
Speckle Removal
The appearance of speckles on the gel may be present after post-electrophoretic staining. To reduce their appearance, cut off the wells before post-electrophoretic staining and rinse the gel in water.
The type and concentration of agarose will affect the degree of speckling. For example, low melting-point agarose tends to be highly prone to speckling.