Revert™ 520 Total Protein Stain
Components
P/N |
Description |
926-10010 |
Revert 520 Total Protein Stain Kit, 200 mLRevert 520 Total Protein Stain, 200 mL Revert Wash Solution, 400 mL Revert Destaining Solution, 400 mL |
926-10016 |
Revert 520 Total Protein Stain Kit, 500 mLRevert 520 Total Protein Stain, 500 mL Revert Wash Solution, 1000 mL Revert Destaining Solution, 1000 mL |
926-10015 |
Revert 520 Total Protein Stain and Wash Solution KitRevert 520 Total Protein Stain, 200 mL Revert Wash Solution, 400 mL |
926-10011 |
Revert 520 Total Protein Stain, 200 mLSufficient reagent for up to 20 mini blots. |
926-10021 |
Revert 520 Total Protein Stain, 500 mLSufficient reagent for up to 50 mini blots. |
Specifications
-
Shelf Life: 6 months from date of receipt
-
Revert Wash Solution: 6.7% (v/v) glacial acetic acid, in water
-
Revert Destaining Solution: 0.1 M sodium hydroxide, 30% (v/v) methanol, in water
-
Membrane Types: Nitrocellulose or PVDF
-
Used to normalize targets detected in the 700 nm and 800 nm channels on the Odyssey M Imager or Odyssey F Imager.
Description
Revert 520 Total Protein Stain is a membrane stain that fluoresces at 520 nm. Total protein stains provide improved quantification accuracy over normalizing to a housekeeping protein, and using the 520 nm channel for normalization enables additional flexibility in your Western blot experimental design. These are some of the specific benefits of using Revert 520 Total Protein Stain for normalization:
-
Accurate quantification: Unlike housekeeping proteins, biological variation won’t affect total protein normalization with Revert 520 Total Protein Stain. Revert 520 Total Protein Stain does not covalently modify sample proteins, so antibody binding and target quantification are not affected.
-
Visible inspection and multiplexing: Stained proteins produce signal that can be seen visibly and by imagers capable of detecting dyes that have excitation and emission maxima near 520 nm. With an imaging system capable of multiplex detection, you can image the total protein stained with Revert 520 Total Protein Stain in the 520 nm channel and image target proteins in the 700 and 800 nm channels on the same blot.
-
Broad linear quantitative range: Revert 520 Total Protein Stain provides high signal intensity with a broad, linear quantitative range.
-
Monitor sample transfer efficiency: With a total protein stain, you will be able to monitor protein transfer across the entire blot at all molecular weights. This will allow you to determine if there are any irregularities that indicate you should run the blot again to get more robust results.
-
Flexible normalization options: Revert 520 Total Protein Stain and Revert 700 Total Protein Stain (licorbio.com/revert) are available so that you can choose the normalization channel that works best for detecting your targets in your particular experiment.
Applications
In Western blot analysis, normalization is essential for accurate, reproducible comparison of protein levels. Validating housekeeping proteins that are suitable for Western blot normalization adds significant time and cost to experiments. The total protein normalization method using Revert 520 Total Protein Stain provides a quick and reliable method for normalizing target signals detected in the 700 nm and 800 nm channels.
Using Revert 520 Total Protein Stain
Use the steps below to stain using Revert 520 Total Protein Stain and to detect targets in the 700 nm and 800 nm channels.
You will image the membrane on an Odyssey M Imager or Odyssey F Imager twice in this protocol: once in step 2 and once in step 5. The first acquisition will be of Revert 520 Total Protein Stain by itself. You will then remove the total protein stain and acquire an image of your targets in the 700 and 800 nm channels.
Method 1: Total Protein Detection Before Western Blot Detection
Step 1. Stain with Revert™ 520 Total Protein Stain
- Add methanol to the stain reagents as indicated on each bottle.
-
After transfer is complete, fully dry the membrane. Place the membrane on top of a piece of clean filter paper and allow it to dry by choosing one of the following:
-
40 to 60 minutes at room temperature.
-
10 minutes in an oven at 37 °C.
-
Overnight at room temperature as a stopping point.
-
-
Rehydrate the membrane after fully drying.
-
For nitrocellulose membranes, incubate the membrane in TBS or PBS (no detergent) for 5 minutes at room temperature with gentle shaking.
-
For PVDF membranes, first rehydrate using 100% methanol for 30 seconds. Then rinse in TBS or PBS (no detergent) for 5 minutes at room temperature with gentle shaking.
-
-
Rinse the membrane with ultrapure water.
-
Stain the membrane with Revert 520 Total Protein Stain. Incubate the membrane in 10 mL of Revert 520 Total Protein Stain solution for 5 minutes at room temperature with gentle shaking.
-
Decant the total protein stain solution thoroughly. Using approximately 10 mL of Revert Wash Solution for each wash, rinse the membrane two times for 30 seconds at room temperature with gentle shaking.
-
Decant the wash solution thoroughly, then briefly rinse the membrane with ultrapure water.
Do not allow the membrane to dry from this point on.
Before moving to the next step, ensure the membrane container provides a minimum clearance of 1/8th of an inch on all sides. Revert 520 Total Protein Stain will cause the membrane to swell. Without clearance, staining may be uneven.
For more information about the Revert protocol, see licorbio.com/revert.
Step 2. Acquire Revert 520 Total Protein Stain Image
-
Do not allow the membrane to dry during imaging. To prevent drying, you may add ultrapure water on top of the membrane.
-
Immediately image the membrane in the 520 nm channel.
See Imaging the Blot in LI‑COR® Acquisition Software for more information.
Step 3. Destaining
The destaining procedure must be performed for Revert 520 Total Protein Stain.
-
After the first imaging, briefly rinse the membrane with ultrapure water.
This step is important for the destaining step to work properly.
-
Incubate the membrane in 10 mL of Revert Destaining Solution for 5 to 10 minutes, with gentle shaking. Destaining is complete when stain is no longer visible by eye.
Do not destain for longer than 10 minutes.
-
Decant the destaining solution thoroughly, then briefly rinse the membrane with ultrapure water.
Proceed immediately to blocking and immunodetection.
Step 4. Proceed with Western Blot Protocol
Follow your normal Western blot protocol using IRDye® 800CW Secondary Antibody to detect your target in the 800 nm channel and IRDye 680RD Secondary Antibody to detect your target in the 700 nm channel.
It is recommended to use 700 nm channel detection for your most abundant target and the 800 nm channel for weak or low abundance targets.
Step 5. Acquire Image for Target Channel(s)
Image the membrane in the 700 and 800 nm channels. Instructions for imaging are provided in Imaging the Blot in LI‑COR® Acquisition Software.
After destaining and Western blot processing, residual fluorescence may be detected in the 520 and 700 nm channel of less than 2%.
Method 2: Total Protein Detection After Western Blot Detection
This method is useful if total protein staining is desired, but not performed on the membrane prior to Western blot detection.
You will image the membrane two times in this protocol, once in step 2 and once in step 5. The first acquisition will be of the target proteins in the 700 and/or 800 channels. The second acquisition will be of the Revert 520 Total Protein Stain.
This method only works if the membrane is processed with a protein-free blocking buffer and antibody diluent.
Step 1. Process Western Blot with Protein-Free Antibody Diluent and Blocking Buffer
Block membrane in Intercept® Protein-Free Blocking Buffer and use Intercept Protein-Free Antibody Diluent for primary and secondary antibody incubations.
Step 2. Acquire Image for Target Channel(s)
Image membrane to capture 700 and 800 nm detection channels.
Step 3. Rinse Membrane with Ultrapure Water
Rinse the membrane with ultrapure water.
Before moving to the next step, ensure the membrane container provides a minimum clearance of 1/8th of an inch on all sides. Revert 520 Total Protein Stain will cause the membrane to swell. Without clearance, staining may be uneven.
Step 4. Stain with Revert 520 Total Protein Stain
-
Add methanol to the stain reagents as indicated on each bottle.
-
Stain the membrane with Revert 520 Total Protein Stain. Incubate the membrane in 10 mL of Revert 520 Total Protein Stain solution for 5 minutes at room temperature with gentle shaking.
Do not allow the membrane to dry from this point on.
-
Decant the total protein stain solution thoroughly. Using approximately 10 mL of Revert Wash Solution for each wash, rinse the membrane two times for 30 seconds at room temperature with gentle shaking.
-
Decant the wash solution thoroughly, then briefly rinse the membrane with ultrapure water.
Step 5. Acquire Revert 520 Total Protein Stain Image
Do not allow the membrane to dry during imaging. To prevent drying, you may add ultrapure water on top of the membrane.
Immediately image the membrane in the 520 nm channel.
Imaging the Blot in LI‑COR® Acquisition Software
Follow the instructions below to image your Western blot membrane using LI‑COR Acquisition Software.
-
Start LI‑COR Acquisition Software
 .
. -
From the Getting Started page, choose Scan.

-
On the Connect page, ensure your username is selected and choose the Odyssey M Imager or Odyssey F Imager that you want to use from the Imager list.
Click Connect.
-
Choose Membrane on the Choose Assay page.

Choosing the appropriate assay will ensure that the image is available for the correct analysis workflow in Empiria Studio® Software.
-
Create a Scan Area for each blot that you want to image by drawing a rectangle around the location of each blot on the Scan Surface.
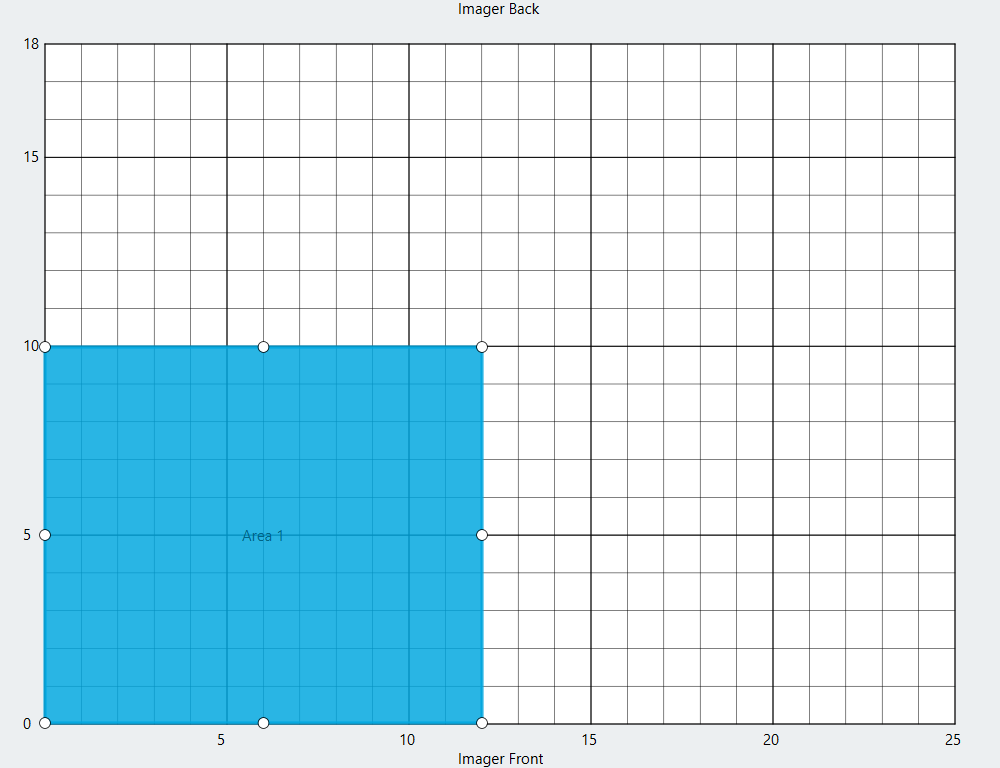
Click Next.
-
On the Detection Options page, click the Select Channels button beside each Scan Area. For each, be sure to select the detection channels you will be imaging.
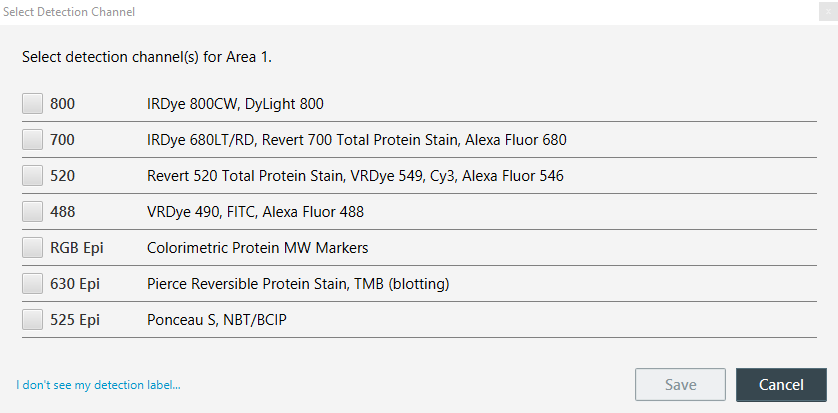
-
Click Scan to begin the scan.
Western Blot Analysis Using Empiria Studio® Software
Empiria Studio Software is designed for reliable analysis of near-infrared Western blots using publisher guidelines. Create a new experiment in Empiria Studio, then choose the appropriate workflow: Linear Range Validation with Total Protein Stain or Target Analysis with Total Protein Stain. Follow the steps provided in the workflow.
Empiria Studio uses workflows to minimize user-to-user variation, provides more extensive analysis options, and can compute vital statistical values. To get started with Empiria Studio, visit licorbio.com/empiria-support.
Analyzing Blots in Empiria Studio Software
-
Start Empiria Studio Software
.
-
On the Home page, click Image Gallery.

-
From the Image Gallery, use the Import LI-COR Zip Files option at the top of the Image Gallery to import the Image Acquisitions from the 520 nm, 700 nm, and 800 nm detection channels.
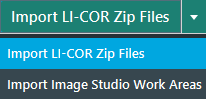
-
After importing the Acquisitions, navigate to the Project
where you would like to save the analysis.
If you have not yet created a Project, you will first need to create one by clicking Create New Project.
-
Click Create New Experiment and choose Quantitative Western Blot or Qualitative Western Blot analysis.


Quantitative Western Blot Experiments include analysis capabilities for comprehensive Western blot projects that include fold change calculations and statistical calculations.
Qualitative Western Blot Experiments provide quick quantification data and charts. Quantitative Western Blot Experiments are similar to Qualitative Western Blot Experiments, but provide additional calculations that you may not need for quick band quantification.
-
Follow the workflow provided by Empiria Studio to complete your analysis.
Related Products
| 926-11011 | Revert™ 700 Total Protein Stain |
| 927‑60001 | Intercept® (TBS) Blocking Buffer |
| 927‑70001 | Intercept (PBS) Blocking Buffer |
| 927‑80001 | Intercept (TBS) Protein-Free Blocking Buffer |
| 927‑90001 | Intercept (PBS) Protein-Free Blocking Buffer |
| 927-65001 | Intercept T20 (TBS) Antibody Diluent |
| 927-75001 | Intercept T20 (PBS) Antibody Diluent |
| 927-85001 | Intercept T20 (TBS) Protein-Free Antibody Diluent |
| 927-95001 | Intercept T20 (PBS) Protein-Free Antibody Diluent |
| 928‑60000 | Chameleon® Duo Pre-stained Protein Ladder (for visual and two-color near-infrared detection) |
| 926‑31090 | Odyssey Nitrocellulose Membranes |
| 928‑40004 | 4X Protein Sample Loading Buffer (optimized for use with near-infrared detection) |